Extrahepatic Shunts for Vets
Anatomy and physiology
Congenital portosystemic shunts (CPSS) are functional communications between the hepatic portal vein and the systemic circulation. In extrahepatic CPSS the abnormal vessel lies outside of the liver. Extrahepatic shunts have a wide range of different morphologies but can be easily characterized by their insertion on the vena cava, azygous vein, or phrenic vein. A CPSS allows blood from the portal system to bypass the liver, resulting in a hypoplastic, underdeveloped liver and effective hepatic insufficiency. The amount of shunting is variable between animals, and this may, in part, reflect the marked variations in clinical signs seen.
History and clinical signs
Overall, extrahepatic CPSS are typically seen in small breed dogs and certain pedigree breeds appear to be predisposed, including Yorkshire terriers, Cairn terriers, West Highland White Terriers and Maltese terriers. As this is a congenital condition, the majority of animals show clinical signs early in life and are typically diagnosed between six weeks and six months of age. Clinical signs are directly related to the shunting of blood past the liver and are characteristic of hepatic insufficiency. This can result in signs relating to the neurologic system, gastrointestinal system, and the urinary tract. Neurologic signs are caused by hepatic encephalopathy (HE) and these are the most common signs seen. Signs of HE can be intermittent and variable. Urinary tract signs are primarily related to ammonium urate urolithiasis as a result of excretion of excessive ammonia. Clinical signs can vary considerably in nature and severity between individuals. Some animals can be presented as emergencies due to severe HE with seizures or even coma, or with dysuria secondary to urethral obstruction from ammonium urate stones. Although most animals show obvious clinical signs at an early age, a small proportion have relatively mild or no obvious signs. Occasionally, animals are diagnosed with a CPSS when mature, either as an incidental finding or due to the development of late onset clinical signs. Decision making for these animals can be challenging.
Diagnosis
Laboratory investigations Routine biochemistry and complete blood count will often show characteristic changes, including anaemia with microcytosis, hypoalbuminaemia, decreased urea, hypocholesterolemia, hypoglycemia and increased liver enzymes. However, these can be variable and are relatively non-specific. Urinalysis will often show decreased urine specific gravity and ammonium biurate crystalluria is seen in some dogs. Liver function tests, including plasma ammonia and dynamic bile acid concentrations are recommended. Bile acids are preferred as they are more accurate and a more readily available. The sensitivity of serum bile acids for CPSS diagnosis is excellent (100% for paired pre- and post-prandial sample analysis), but they cannot be used alone to differentiate a CPSS from other potential causes of liver dysfunction. Occasionally, animals may have normal pre-prandial serum bile acids concentrations and a dynamic test is recommended.
Diagnostic imaging
Diagnostic imaging is necessary to identify the shunt in order to confirm the diagnosis and to rule out other causes of liver dysfunction. It also provides important information on the location and morphology of the shunt to allow appropriate treatment. Abdominal ultrasonography is useful as a screening tool and may provide sufficient information on the nature of the shunt for surgical planning. Although convenient, ultrasound is very operator dependent, and the accuracy is therefore variable. Computed tomography angiography (CTA) is recommended as the gold standard for imaging CPSS and provides a wealth of information on the location and morphology of the shunt as well as the degree of portal vasculature development. It is minimally invasive and can be performed safely prior to surgery but is relatively expensive and does require sedation or anesthesia. In our experience, ultrasonography, performed by an experienced operator is sufficient to diagnose a shunt and allow surgical planning in most animals with extrahepatic CPSS.
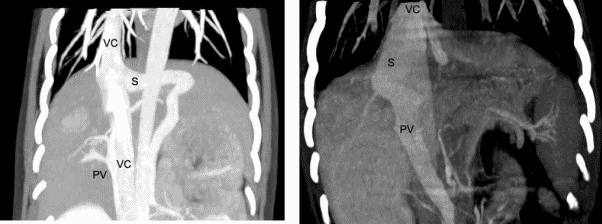
(right). S = shunt, PV = portal vein, VC = vena cava
Stabilisation and medical management
The initial management for dogs diagnosed with CPSS is medical. Medical management aims to treat the signs of HE and consists of a combination of lactulose, low-protein diet, and antimicrobials. Medical management can be used as the sole treatment for dogs and cats with CPSS and can result in an acceptable long-term outcome. However, medical management does not address the underlying cause or improve liver function. Therefore, dogs are at risk of recurrent clinical signs and their quality of life may not be normal. Decision Making In general, surgical management is recommended for dogs with extrahepatic CPSS. Successful surgery should give the affected dog the best possible long-term outcome in terms of survival and quality of life. However, surgery is not without risks and this must be considered when determining the best treatment for an individual. Medical management can be used long-term for animals where surgery is not considered appropriate or when it is declined by the owner. Regardless, a period of two to four
weeks of medical management is recommended to stabilize the animal prior to surgical shunt attenuation. Occasionally, animals with minimal or no clinical signs can be diagnosed with a CPSS, often when mature. Deciding between medical and surgical management (or even no treatment for animals without clinical signs) can be challenging. Surgical management is associated with a risk of significant complications and mortality, which are likely to be increased in older animals. It can also be difficult to assess the cost / benefit of surgery for animals with no apparent clinical signs.
Surgical management
The aim of surgery is to close the shunt and restore normal portal blood flow to the liver and hence normal liver function. In an ideal world, all shunts would be completely closed at the time of surgery. However, complete shunt closure is not possible in approximately 50% of dogs with extrahepatic CPSS as the liver and portal venous system are hypoplastic and cannot tolerate normal portal flow leading to portal hypertension. For this reason, gradual attenuation devices have been developed to allow complete shunt closure without the risk of portal hypertension. These include ameroid constrictors and thin film bands. These are placed around the shunt and provide gradual closure over a period of weeks. However, one disadvantage of these devices is that once they have been placed there is no control over the rate or completeness of closure. This can lead to persistent shunting if the device fails to close the shunt completely or if the shunt closes too rapidly, resulting in MAS. Persistent shunting is reported in 24- 37.5% of dogs. At Paragon we tailor the surgical treatment to the individual dog and their owners. We believe that the overall goal of shunt surgery should be to completely close the abnormal vessel either gradually or acutely, in order to abolish all shunting and give the dog the best possible outcome. We perform intraoperative mesenteric portovenography in all dogs during surgery to help identify the shunt and assess the degree of portal development. Dogs are assessed during the surgery to see if they can cope with full closure of the shunt (based on subjective and objective assessment of portal pressures and the degree of portal development on IOMP). Complete ligation is performed in dogs that can tolerate it and those that can’t have placement of an ameroid constrictor or thin film band. A length of suture is left around the shunt in order to facilitate any future surgery if persistent shunting is subsequently identified.
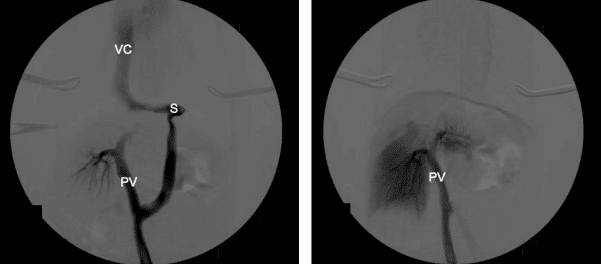
congenital portosystemic shunt (CPSS). The image on the left shows the majority of blood flow is
through the shunt, with some opacification of the portal vein and intrahepatic vasculature. The image on the right shows increased opacification of the intrahepatic vasculature and no contrast flow through
the shunt, following complete closure. VC = vena cava, PV = portal vein, S = shunt.
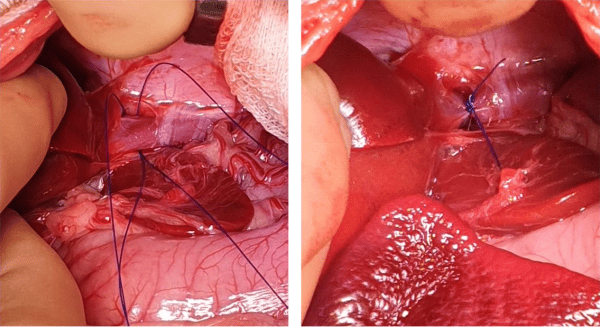
cava and two loops of polypropylene suture have been placed around it. On the right the shunt has
been completely ligated.
Complications and Postoperative Care
Attenuation of a CPSS has been associated with significant complications, including post-attenuation neurological signs (PANS), portal hypertension, peri-operative haemorrhage and hypoglycemia. PANS are the most significant post-operative complications in dogs with extrahepatic shunts. The cause of PANS is unclear, although it is thought to be related to changes in aberrant neurotransmitters associated with HE. PANS vary in severity from mild tremors, ataxia, or changes in behavior to blindness or seizures. Increased age has been associated with significant increased risk of PANS. Overall, the mortality rate for surgical treatment of dogs with extrahepatic CPSS is approximately 5%, and this is mostly due to PANS.
Prognosis and Long-term Follow-up
When successful, surgery results in improvement in portal blood flow and liver volume, due to hepatic regeneration. This results in improvement in liver function and hence resolution of clinical signs. We typically recommend that dogs remain on medical treatment post-operatively. Repeat blood tests, including bile acids are normally performed 8-12 weeks following surgery. Assuming that bile acids have normalized dogs can stop medical treatment and be treated normally.
Some dogs have a persistent mild increase in bile acids following surgery, despite a good clinical outcome and this is not a problem. However, significantly increased serum bile acid concentrations are not expected post-surgery and suggest persistent shunting. Dogs can have persistent shunting due to incomplete closure of the CPSS or due to the development of MAS, which can cause ongoing or recurrent clinical signs. Dogs with clinical signs post-surgery should undergo further imaging to assess for persistent shunting. Animals with persistent flow through the CPSS are likely to benefit from repeat surgery to attempt further closure. Long-term medical management can be considered as an alternative, or for animals with MAS. Nevertheless, some dogs can have a good clinical outcome despite significant persistent shunting.
